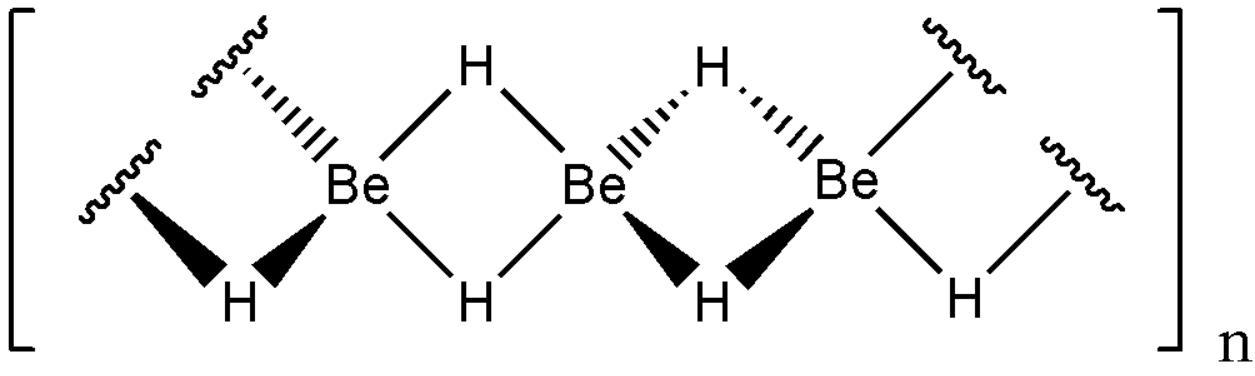
Beryllium hydride (officially known as poly [beryllan (2)] and beryllium dihydrogen) is an inorganic compound with the chemical formula of (beh2) n (also known as ([beh2]) n or beh2). This alkaline earth hydride is a colorless solid, insoluble in solvents and does not decompose. Unlike the ionic bond hydrides of the heavier second group elements, beryllium hydride is formed by covalent bonds (three center double electron bonds).
Unlike other second group metals, beryllium does not react with hydrogen. In contrast, beh2 was prepared from pre formed beryllium (II) compounds. In 1951, dimethyl beryllium be (CH3) 2 was first synthesized with lithium aluminum hydride (LiAlH4). The relatively pure beh2 was prepared by pyrolysis of Di tert butyl beryllium be (C (CH3) 3) 2 at 210 ℃. The formation of high purity samples in route a involves the reaction of triphenylphosphine PPh3 with beryllium borohydride be (BH4) 2. Be (BH4) 2+2pph3 → beh2+2ph3ph3
The isolated beh2 molecule (sometimes called beryllium dihydrogen or written as [beh2] to emphasize its difference from solid state) is stable only as an inert gas. After concentration, the nonsolvated beh2 was spontaneously polymerized. The free molecule beh2 produced by high temperature discharge has a linear geometry, and the be-h bond length is 133.376 PM. The cross is sp.
Beh2 is usually formed in the form of amorphous white solid, but using 0.5-2.5% LiH as catalyst, heating amorphous beh2 under pressure can obtain hexagonal structure with higher density. The crystalline form (~0.78 gcm-3) has been reported. In a recent study, crystalline beryllium hydride contained a beh4 tetrahedron with a shared angle, rather than a flat, hydrogen bridged infinite chain previously thought to exist in crystalline beh2. It is found that it has a volume centered orthogonal cell containing a network. We also find that the amorphous form is composed of a tetrahedral network sharing an angle.
Beryllium hydride reacts slowly with water, but is rapidly hydrolyzed by acids such as hydrogen chloride to form beryllium chloride. Beh2+2H2O → be (OH) 2+2h2beh2+2HCl → BeCl2+2h2
The 2-hydroxy beryllium group can accept the electron ligand (L) in the molecule by addition reaction. [beh2]+L → [beh2l] because these reactions are energetically advantageous, beryllium hydride has Lewis acid properties. Libeh3 and li2beh4 are successively generated by reacting with lithium hydride (where the hydrogenated ion is Lewis base). The latter contains tetrahydropinaric acid (2-) anion beh2-4. Beryllium hydride reacts with trimethylamine n (CH3) 3 to form a dimer adduct with bridged hydride. However, when dimethylamine, HN (CH3) 2, is used, the trimer beryllium diamine [be (n (CH3) 2) 2] 3 and hydrogen are formed.