Sodium hydroxide (NaOH) is widely used in the production of paper, soap, dyes, artificial silk, metal smelting, petroleum refining, cotton fabric finishing, purification of coal tar products, food processing, wood processing, and machinery industries
Sodium hydroxide is widely used in the national economy, and many industrial sectors require it. The department that uses sodium hydroxide the most is the manufacturing of chemicals, followed by paper making, aluminum smelting, tungsten smelting, artificial silk, artificial cotton, and soap manufacturing
In addition, a large amount of caustic soda is also used in the production of dyes, plastics, chemicals, and organic intermediates, as well as in the regeneration of old rubber, the electrolysis of metal sodium, water, and inorganic salt production, such as the production of borax, chromium salt, manganese salt, phosphate, etc
Meanwhile, sodium hydroxide is one of the important raw materials for the production of polycarbonate, super absorbent polymers, zeolite, epoxy resin, sodium phosphate, sodium sulfite, and a large amount of sodium salts
Sodium hydroxide is a white translucent crystalline solid, and its aqueous solution has an astringent and greasy taste
Water absorption (deliquescence): Sodium hydroxide is prone to deliquescence in the air, so solid sodium hydroxide is commonly used as a desiccant. However, liquid sodium hydroxide does not have water absorption
Solubility: It is highly soluble in water and releases a large amount of heat when dissolved. It is easily soluble in ethanol and glycerol
1. Can be used as a chemical experiment. In addition to reagents, it can also be used as an alkaline desiccant because it has strong water absorption and deliquescence. It can also absorb acidic gases, for example, in experiments where sulfur burns in oxygen, sodium hydroxide solution can be placed in a bottle to absorb toxic sulfur dioxide.
3. The characteristics of sodium hydroxide determine that this product is an indispensable and important substance in a large number of chemical reactions. Sodium hydroxide is one of the important raw materials for producing polycarbonate, super absorbent polymers, zeolites, epoxy resins, sodium phosphate, sodium sulfite, and a large amount of sodium salts.
4. Sodium hydroxide plays an important role in the papermaking industry. Due to its alkaline properties, it is used for boiling and bleaching paper pages.
5. Sodium hydroxide can be widely used in the production of starch, preparation of carboxymethyl cellulose, and manufacturing of sodium glutamate.
6. Sodium hydroxide is widely used in water treatment. In sewage treatment plants, sodium hydroxide can reduce the hardness of water through neutralization reactions. In the industrial field, it is a regenerant for ion exchange resin regeneration. Sodium hydroxide is highly alkaline and soluble in water. Due to the relatively high solubility of sodium hydroxide in water, it is easy to measure dosage and use in various fields of water treatment.
Sponsored
One of the chemical products Sodium hydroxide knowledge introduction
Posted 2023-08-17 14:03:24
0
34
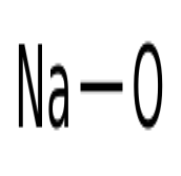
Search
Sponsored
Categories
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
Read More
Book Bed and Breakfast
Are you planning your next getaway and searching for the perfect holiday accommodation? Look no...
Dermatology EMR Software Market Technology and Opportunities Forecast
"Dermatology EMR Software Market Size" research report looks at the main drivers impacting global...
Cafe in Mussoorie and Your Gateway to Serene Nature
In India's Uttarakhand state, Mussoorie is a charming hill town that is tucked away in the...
Let's Keto Gummies South Africa – Real Or Fake Weight Loss Gummies – User Reviews
They passed on a thing called Let's Keto Gummies which is needed to condition...
Do You Make These Simple Mistakes In Sap C_c4h320_02 Exam Dumps?
SAP C_C4H320_02 Exam Dumps We provide the whole lot you need up-to-date pinnacleupdated the SAP...
Sponsored